Nancy, a 26-year-old women from a remote region, G1P0, is referred to you at 35 weeks gestation for opinion regarding anaesthetic for her delivery plan. She has a history of balloon mitral valvuloplasty at 21 weeks gestation and is currently taking Clexane 40mg daily for thrombus prophylaxis. She desires to have a vaginal birth.
Candidate has 2 minutes reading time to plan their initial response to the first question below
Click each question to reveal a suggested answer
OPENING QUESTION
1. What further key information would you actively seek before proceeding with any management decisions?
Balloon valvuloplasty for the mitral valve is obviously a highly unusual procedure for a pregnant woman. The candidate should immediately demonstrate an appreciation of this fact and suspicion that the patient has moderate to severe mitral stenosis.
This should automatically prompt the candidate to seek further information regarding mitral stenosis and potential associated complications followed by a standard obstetric assessment.
History
Why did she have a balloon valvuloplasty? Presume a diagnosis of mitral stenosis - what is the aetiology? Rheumatic heart disease?
Detailed symptoms of heart failure (dyspnoea, orthopnoea, paroxysmal nocturnal dyspnoea, fatigue, angina, syncope), their progression, and current functional capacity (NYHA class) - specifically pre-valvotomy compared with any improvement post-valvuloplasty.
Compliance with Clexane, any prior bleeding or thrombotic events.
Her understanding of her high risk cardiac condition, the valvuloplasty, and the Clexane
Review the anaesthetic record for the valvuloplasty
Examination
Focused cardiac examination for signs mitral stenosis
Assess for signs of heart failure (e.g., raised JVP, pulmonary crackles, peripheral oedema, S3, new murmurs).
Assess for signs of right heart failure (e.g., hepatomegaly)
General condition, vital signs (especially any tachycardia, hypotension, or desaturation)
Airway/spine assessment.
Investigations
Baseline ECG - for AF
TTEs (pre- and post-valvuloplasty) - MV function, pulmonary arterial pressures, LV and RV function.
Cardiac biomarkers (e.g. NT-proBNP to differentiate normal pregnancy symptoms from heart failure).
FBC - for any anaemia, thrombocytopaenia
Coagulation profile (INR, aPTT, fibrinogen)
Electrolytes, renal function
Liver function.
Review the obstetric US for any foetal abnormalities or placental implantation issues.
PROGRESS QUESTIONS
2a. Nancy had a balloon valvuloplasty for symptomatic severe mitral stenosis, secondary to rheumatic heart disease. Since 15 weeks gestation, she had dyspnoea on exertion with basic everyday activities including walking a set of stairs. This also triggered some palpitations but she denies angina, orthopnoea, or paroxysmal nocturnal dyspnoea. She is clinically stable with no signs of heart failure on examination and not on any cardiac medications. She has been self-administering Clexane as instructed.
The echocardiogram 2 weeks after valvuloplasty reported these findings:
Mean gradient 11mmHg
Valve area 0.9cm2
Severe biatrial dilatation
Estimated PASP 42 mmHg
LV EF > 60%
2b. Briefly interpret these results
The purpose here is to test the candidate's ability to stratify the severity of mitral stenosis.
A MG > 10mmHg and valve area <1.0cm2 meets criteria for severe mitral stenosis. A pressure half-time is not measured here but if it was ≥220ms, then this would fully consolidate an echocardiographic diagnosis of severe MS.
The significant atrial dilatation and elevated PASP reflect chronic maladaptation to progressive MS and is concerning for likely moderate pulmonary hypertension and potential right heart strain. A normal LV EF is often reassuringly associated with satisfactory cardiac output, however in the setting of known severe MS and typically hyperdynamic contractility, the absolute stroke volume is actually reduced. Therefore, I view the LV EF 60% with caution.
2c. (If not previously volunteered) How do you interpret the ejection fraction of 60% in the setting of these other findings of severe MS?
A normal LV EF is often reassuringly associated with satisfactory cardiac output, however in the setting of known severe MS and typically hyperdynamic contractility, the absolute stroke volume is actually reduced. Therefore, I view the LV EF 60% with caution.
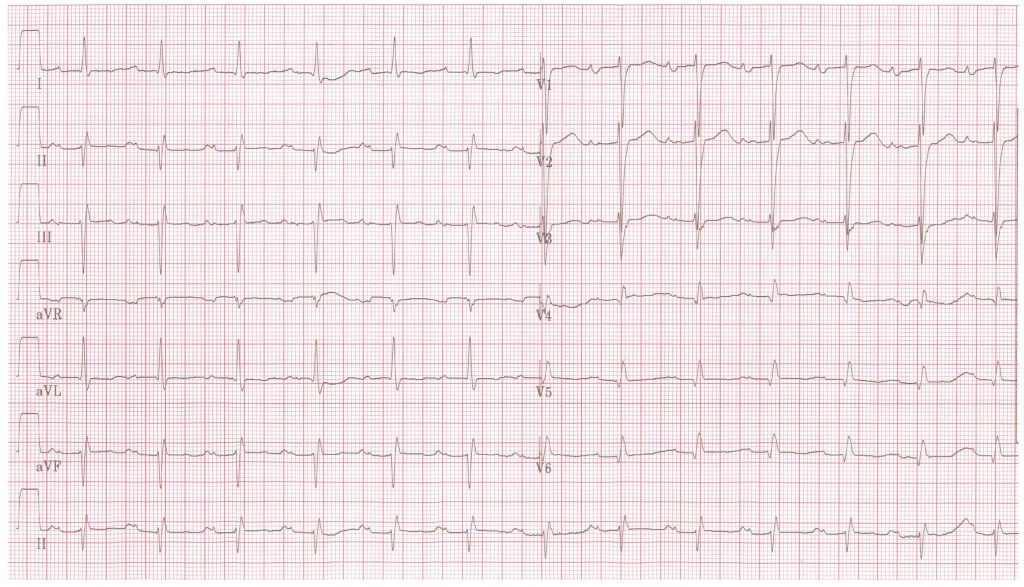
3. Here are the patient’s recent blood tests and ECG [click here] (ideally the candidate should have stated they would request these in their assessment). Please comment
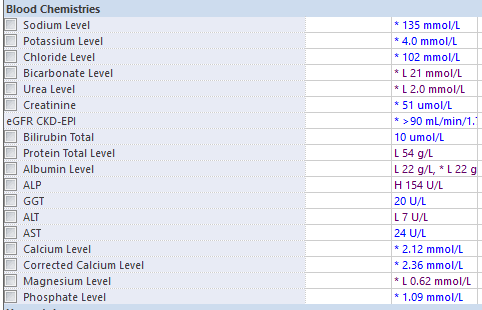
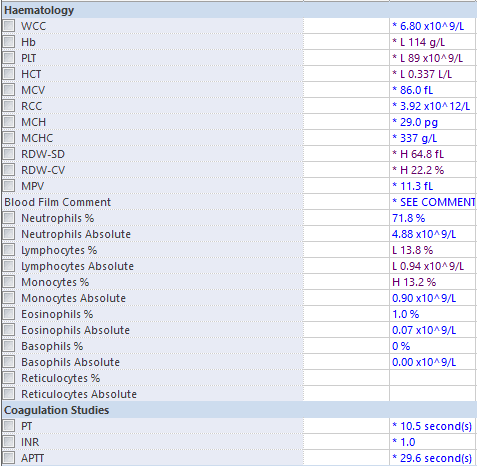
Thrombocytopaenia
Hypomagnesaemia
- these should be compared with previous results to assess the trend.
- if the platelet count deteriorates further it will have implications for neuraxial anaesthesia and managing intrapartum haemorrhage.
ECG:
- P mitrale (bifid P wave) in Lead II.
- Biphasic P wave in V1
- these are both typical findings in MS and match this patient's known atrial dilatation
- there is also a 1st degree AV block
- I would also be looking for features of AF, right heart strain / hypertrophy - common in pulmonary hypertension
4. This patient has multiple complex issues. Who needs to be part of the team in managing this pregnancy and delivery? What are the key discussion points you would bring to that meeting, especially considering her social situation?
Expected response: MDT must include high-risk obstetrician/Maternal-Fetal Medicine (MFM), cardiologist, haematologist, neonatologist and anaesthetist
Discussion Points:
Risk assessment and stratification: Confirm mWHO classification (likely Class IV due to severe MS and PH).
Patient's values and preferences:
- Shared decision-making, acknowledging her preference for vaginal birth but gently emphasising the need for regular re-evaluation and potential for Caeserean delivery depending on clinical stability.
- The challenges of her social vulnerability must be central to all discussions.
- All these discussions and medical optimisation before IOL /surgery - this is crucial.
5. Let’s focus on her anticoagulation and thrombocytopenia. She is on Clexane 40mg daily and her last recorded platelet count was 89. The provisional plan is IOL at 38 weeks. How would you manage her Clexane peripartum and what is your immediate concern and plan for her thrombocytopaenia?
Clexane: she is on a prophylactic dose. For neuraxial analgesia/anaesthesia, needs to be omitted for 12 hours before the block. If a C-section under GA is decided, this timing is less critical, but still relevant for general bleeding risk.
Thrombocytopaenia: Platelet count of 84 is a significant concern and is a relative contraindication to neuraxial anaesthesia if there has been a progressive consistent downtrend. Based on international and local guidelines, a hard minimum platelet count of 70 is commonly accepted. Immediate plan is to repeat the platelet count to confirm accuracy and check for trend. Consult haematology urgently to investigate the cause (e.g., rule out ITP, pre-eclampsia related thrombocytopenia, or other secondary causes) and advise on management, including potential platelet transfusion thresholds.
6. The patient has expressed a strong preference for a vaginal birth. From an anaesthetic point of view, what are the risks and benefits of vaginal birth versus an elective Caesarean section for this specific patient?
Vaginal Birth:
Benefits:
# Less likely PPH
# More gradual haemodynamic changes with carefully titrated epidural analgesia.
Risks:
# Acute heart failure from effects of severe MS/PHTN during labour, particularly towards end of 1st stage and during 2nd stage, even if pain is controlled.
- may occur due to increased cardiac demand, leading to pulmonary congestion and potential right heart failure, especially with pain-mediated tachycardia and Valsalva manoeuvres.
- Valsalva decreases preload and increases afterload, which is detrimental in MS and PHTN.
- Autotransfusion causing the sudden preload increase after delivery can precipitate flash pulmonary oedema.
# The abnormal stenotic valve physiology is compounded further and more complex to manage in the delivery suite environment, if her IOL and labour does not progress uneventfully e.g. prolonged labour, obstructed labour, indications for syntocinon infusion, intrapartum haemorrhage, inadequate epidural block.
# Thrombocytopenia/Anticoagulation: Significant risk of PPH, which is higher in cardiac patients. Contraindication to neuraxial techniques if platelet count continues to fall.
# An assisted delivery is ideal to help shorten second stage to mitigate previously described physiology. This may increase the risk of bleeding secondary to trauma.
Elective Caesarean Section:
Benefits:
# Controlled environment, optimised team, and planned anaesthesia.
# Avoids pain-mediated haemodynamic swings and Valsalva manoeuvres
Risks:
# Generally associated with more blood loss
# Titration of neuraxial technique (spinal, CSE, epidural), or GA for adequate surgical conditions may be unpredictable and precipitate excessive hypotension and inadequate cardiac output.
# However, if performed under GA, it avoids the issues of thrombocytopenia/anticoagulation relevant to the risk of vertebral canal haematoma with neuraxial techniques.
7. Nancy cardiac function remains stable over the next 3 weeks. Haematologist is consulted and diagnoses her with gestational thrombocytopaenia. Her FBC is repeated periodically to monitor her platelet count. After further consultation with Obstetrics she and her partner have agreed for an elective Caesarean Section. She is now 38 weeks and on your LSCS list. Her last clexane dose was 12 hours ago and her platelet count over the last 2 days has been 110 x 10^9/L and 124 x 10^9/L. How do you plan to anaesthetise her?
The candidate should respond professionally with a concise summary of their plan. They should avoid just rattling a list of tasks but rather focus on the goals of the anaesthetic and how to acheive them. Given the platelet count has recovered, and there is no abnormal coagulation, a neuraxial approach is ideal. GA is not incorrect, but there must be strong rationale for it and against a neuraxial.
Goal
Acheive a comfortable awake delivery, preserving maternal-neonatal bonding without haemodynamic compromise
- maintain baseline preload and afterload - limit IV fluids to < 500mL
- strictly defend against tachycardia and pulmonary vasoconstriction
Vigilant monitoring with early detection of hypotension, arrhythmia or heart failure
Superior pain control in the acute post-op period
Close observation of haemodynamics and cardiac function post-op - best suited for ICU
Anaesthetic Mode
Combined Spinal Epidural with a reduced initial spinal dose followed by gradual epidural top up, to achieve surgical anaesthesia for a lower segment uterine incision and a dermatomal block to ice cold at T5.
The back option if this 1st plan fails after unsuccessful epidural top ups is a relaxant GA with an ETT.
Monitoring
In addition to standard ANZCA monitoring:
- arterial line for beat-to-beat BP monitoring
- 5-lead ECG for ischemia detection
- Defib pads pre-placed in event of arrhythmia e.g AF (30 - 40% risk) or at worst cardiac arrest
Access
Central venous line - for inotropes/vasopressors.
2 large bore IVC
Drugs
Uniquely prepared for this case I would also have:
- phenylephrine infusion (100mcg/mL) or metaraminol
- noradrenaline infusion (60mcg/mL)
- oxytocin bolus of 1unit and a separate small volume infusion of 0.8 units/mL (40 units in 50mL) - specifically to avoid excessive IV fluid administration and the risk of pulmonary oedema
- specifically AVOID ergometrine, carboprost due to their significant pulmonary vasoconstricting and systemic effects
specifically AVOID ephedrine due to unwnated chronotropic effects
- frusemide in the event of pulmonary oedema
Extra resources
BiPAP machine
Cardiac anaesthetist on standby to assist with intra-operativeTOE/TTE
8. What dose would you use in your initial spinal and the the epidural top-ups?
There is no specific answer for this. The candidate is expected to give a sensible dose, obviously it would be less than the dose for a typical elective LSCS.
e.g.
0.8mL hyperbaric bupivacaine 0.5%, 20mcg fentanyl, 100mcg morphine.
Top-Ups: 2% lignocaine (plain) 3 - 5mL at a time
9. (If not described previously, prompt): what drugs would you consider avoiding for this patient during the delivery
specifically AVOID ergometrine, carboprost due to their significant pulmonary vasoconstricting and systemic effects
specifically AVOID ephedrine due to unwnated chronotropic effects
CRISIS QUESTIONS
10a. After you complete your CSE you assess your block. At 3 minutes post block she has zero movement in her legs and block to ice cold is at T8. Phenylephrine infusion was started at the administration of the spinal dose. It is running at 800mcg/h. No epidural top-ups have been given yet. Your registrar looks puzzled and asks if you gave the reduced spinal dose as planned. Her vital signs are (click here):
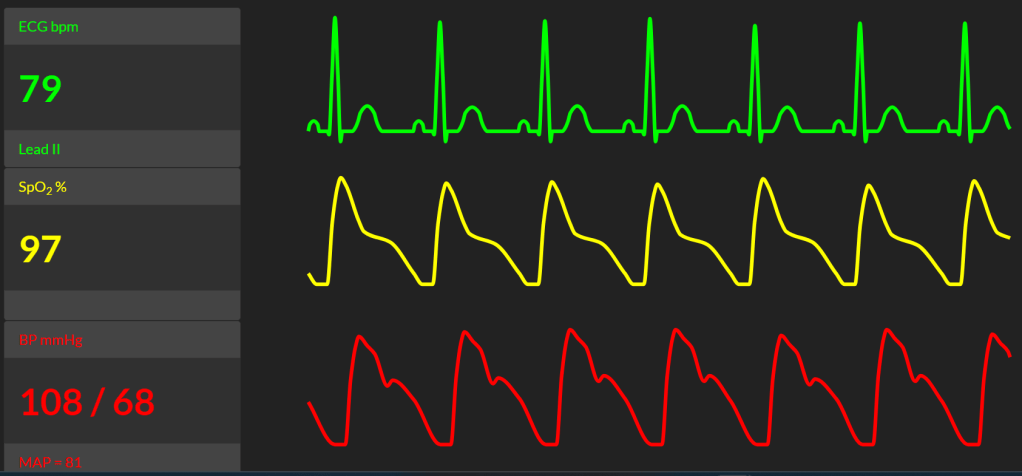
10b. How do you respond to your registrar?
The point here is to ensure the candidate can problem solve this situation logically without panicking. It is an elective procedure in a high risk patient. There is no emergent complication as yet, and there is time to address the issues. Candidates that do no appreciate that a rapid block with a low dose is unusual and don't consider the potential flow on risks will not do well.
This is unexpected to occur with a reduced spinal dose
Differential diagnosis :
- medication error (any time point from LA selection to drawing up dose)
- administration error (rapid injection, excessive barbotage)
- testing error (poor understanding from patient and inaccurate subjective reporting)
- significantly narrowed subarachnoid space secondary to gravid uterus
Risks:
- high spinal
- severe hypotension and cardiac failure
Action:
1. Reassess patient. Ask how they are feeling. Specifically nausea, lightheadedness, dizziness, drowsiness
2. Re-check dermatomal block with light touch and sharp pinch
3. Have ventilation circuit and mask plus induction drugs ready in event of high spinal
4. Keep patient, partner and Obstetrics team informed
11. You wait 5 minutes and reassess the block. Dermatomal height remains at T8. How do you proceed?
Slowly titrate up epidural and reassess
e.g. 4mL of 2 % lignocaine
12. Your top-up works and the surgeons make a start. Midway through the Caesarean section, the patient complains of difficulty breathing and feeling faint. She denies any pain. What is your differential diagnosis and how would you manage this acute change? (Click here to see her new observations & suggested response)
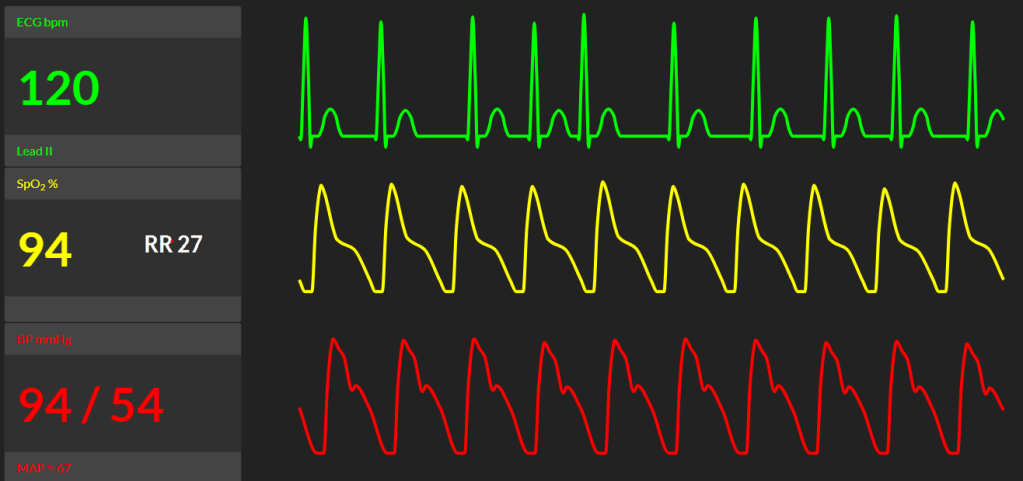
Differential diagnosis
This clinical change is concerning. The top priority is to assess for acute pulmonary oedema and evolving heart failure. Other differentials include:
High Neuraxial Block
Block started higher than anticipated. Epidural top ups may have caused an excessive ascending block causing respiratory muscle weakness and/or cardiac dysarrhythmia.
Atrial fibrillation:
ECG check. Manage as per ACLS for pregnant patient.
Pulmonary Embolism:
Increased risk in PH and hypercoagulable pregnancy.
Signs: acute desaturation, RV strain on echo.
Valve Thrombosis/Obstruction:
Could manifest as acute stenosis or regurgitation, leading to heart failure.
Signs: sudden increase in valve gradient, decrease in valve area/mobility, new murmur.
Haemorrhage:
Rule out obstetric bleeding. Check with surgeons, inspect suction canister.
Management
Assess patient:
A-B-C-D
Ensure secure/patent airway,
Auscultate chest
Provide 100% O2 with manual CPAP via ventilation circuit with APL set to 5 cmH2O to start
Haemodynamics: Confirm arterial line transducer is accurately positioned.
Bolus phenylephrine (e.g 50mcg) and increase infusion rate.
Cardiac rhythm has become irregular - feel pulse to confirm if it's AF
Connect defib pabs in preparation for DC cardioversion if AF becomes unstable
Check height of block with ice / sharp pinch
13. On auscultation, bilateral crepitations are heard in the lung fields. She feels some relieve from the oxygen and CPAP you provide (if candidate didn’t use anaesthetic circuit mask in this way or provide CPAP in some form, prompt them). Her blood pressure improves to a MAP of 74 mmHg, SpO2 to 97% but her heart rhythm remains in AF, heart rate 127 bpm. The obstetrician announces she is about to deliver the foetal head. What do you do now?
Priorities now include:
- Expedited delivery of foetus
- Rate control of AF
- Maintain preload and afterload without worsening pulmonary oedema
The patient needs beta-blockade. Esmolol loading and infusion would be the ideal treatment - given it's cardioselective and rapidly acting. However there is minimal data in literature on it's effect on neonatal cardiac function in the immediate short term. Concern would be bradycardia from placental transference. At worst, heart block.
The risk of teratogenicity is more complex. Starting a dose at the time of immediate delivery is likely to have negligible impact on neonatal development especially in a term baby. However, there is no data on this and consultation with cardiology in real-time is warranted.
Overall, the benefits of antiarrhythmic therapy outweigh the risks.
Given haemodynamic stability has been acheived, I would wait till the baby is delivered before administering treatment.
14. The obstetrician delivers the baby uneventfully. The midwife helps Nancy have a brief cuddle and then performs a health check at the resuscitaire. The obstetrician calls for uterotonics. What is your uterotonic strategy (if not volunteered previously. Otherwise move on to next question)?
Oxytocin bolus 1 unit
Then infusion of 10 units oxytocin / hour (if 0.8 units/mL, 10u/h = 12.5mL/h)
+/- Misoprostol 600 - 100mcg SL/PV/PR
15. Nancy remains in AF with rapid ventricular response. How do you respond to this?
Reassess observations / vital signs
Inform patient and surgical team
Continue phenylephrine infusion titrating for BP within 10 - 20% of baseline
Start rate control therapy with high vigilance on BP and avoiding sudden hypotension or bradycardia
Esmolol
Initial bolus:
500 mcg/kg IV over 1 minute.
Infusion:
Start at 50 mcg/kg/min.
Titrate every 5–10 minutes up to 200 mcg/kg/min based on HR and BP.
Regularly check volume status - urine output, blood loss - and uterine tone with the surgeons. Haemorrhage or hypovolaemia would worsen AF.
Alternative Rx could be satisfactory provided candidate gives correct doses and also recognises caveats:
- IV Metoprolol: 2.5 - 15mg. Class I recommendation. Likely require multiple boluses. Can't give as an infusion
- IV Digoxin: Class IIa option. Slower onset, useful in hypotensive patients.
- IV Verapamil : Class IIa option. May also result in chemical cardioversion.
- IV flecainide / Ibutilide: only suitable if termination of AF / rhythm control desired and not suitable for structural heart disease
- Amiodarone / Diltiazem: Avoid in pregnancy due to potential fetal effects and hypotension.
AFTERMATH QUESTIONS
16. Your treatment is successful. Heart rate improves to 90 bpm. The patient asks if your registrar can stop holding the anaesthetic circuit mask on her face. What do you do?
The patient has likely suffered flash APO which she was at high risk of in the context of severe mitral stenosis. She is also dependent on phenylephrine infusion and on an oxytocin infusion.
She requires ongoing support for reducing cardiac preload and maintaining steady afterload. This is best achieved with NIV, judicious diuretics and gradual titration and weaning of vasopressors, in the ICU.
I would advise Nancy, her partner and the surgical team of this plan. Tactfully apologise that she can have a very brief break from the mask but then would need to change to a proper bilevel NIV mask on transfer to ICU.
Actions:
Refer patient to ICU
Prepare for transfer with NIV, oxygen, backup vasopressor infusion
17. (if not volunteered previously) The ICU has accepted Nancy but is currently full. They advise to start NIV in PACU in the interim. Your nurses are not familiar with setting NIV and ask you for help.
There is no high expectation for that candidate to know in depth how to operate NIV machines. However, the principles of the basics is expected.
Confirm patient is compliant / agreeable
Connect to wall oxygen
Ensure mask has firm seal
Settings:
IPAP: Start at 10–15 cm H₂O
EPAP: Start at 5 cm H₂O
FiO₂: Titrate to maintain SpO₂ > 94%
Backup rate: Optional; consider 12–16 bpm if fatigue is present
Off-mask breaks: depends on severity of respiratory failure. May consider for oral fluid intake.
Consult with Respiratory Physician / CNC / ICU to guide NIV plan
END OF VIVA
Sources
Title Featured Image https://anaesthesianews.wordpress.com/wp-content/uploads/2018/02/mitral-valve-stenosis.jpg
2025 ESC Guidelines for the management of cardiovascular disease and pregnancy. European Heart Journal. 00, 1–107 https://doi.org/10.1093/eurheartj/ehaf193
ECG https://en.ecgpedia.org/wiki/Main_Page
Arjun, Manickavasagan R, Praveen P. Epidural Anaesthesia a Saviour in a Case of Mitral Stenosis Posted For Emergency LSCS. Ann. Int. Med. Den. Res. 2019; 5(5):AN17-AN18. https://aimdrjournal.com/wp-content/uploads/2021/07/AN5_CR_Arjun-1-edit.pdf
Mitral Stenosis – Ten Rules for Anesthesia Considerations. Anaesthesia News. https://anaesthesianews.wordpress.com/2018/02/12/mitral-stenosis-ten-rules-for-anesthesia-considerations/
Krugh M, Patel P, Maani CV. Misoprostol. [Updated 2024 Dec 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539873/
2023 Guideline for the Diagnosis and Management of Atrial Fibrillation. Circulation. 2024;149:e1–e156. https://doi.org/10.1161/CIR.0000000000001193
Youssef, Ghada Sayed. Management of atrial fibrillation during pregnancy. e-Journal of Cardiology Practice. Vol. 17, N° 15 - 17 Jul 2019. https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-17/management-of-atrial-fibrillation-during-pregnancy#
Bello G, De Santis P, Antonelli M. Non-invasive ventilation in cardiogenic pulmonary edema. Ann Transl Med. 2018;6(18):355. doi:10.21037/atm.2018.04.39
Nickson, Chris. Non-Invasive Ventilation (NIV). LITFL. October 2024. https://litfl.com/non-invasive-ventilation-niv/